.button {
float: left;
width: 100%;
padding: 10px 15px;
margin: 0 10px 20px 0;
color: #fff;
font-size: 14px;
text-transform: uppercase;
text-align: center;
border-radius: 3px;
background: #c4c5c5;
}
.button:hover {
color: #fff;
}
.button.blue {
background: #7ea7cc;
}
.button.blue:hover {
background: #9fbed8;
}
.button.green {
background: #6e8428;
}
.button.green:hover {
background: #93a261;
}
.three-column {
float: left;
width: 31%;
margin: 0 2% 40px 0;
}
.three-column.last {
margin-right: 0;
}
.anchor-nav .btn {
padding: 5px 20px;
margin-right: 10px;
}
.anchor-nav .btn.active {
background: #446b8f;
}
.form-field.half {
padding-bottom: 0;
}
@media screen and ( max-width: 720px ) {
.three-column {
width: 100%;
margin-left: 0;
margin-right: 0;
}
.three-column.buttons {
margin-bottom: 20px;
}
.banner {
display: none;
}
}



Please submit the required fields to be connected with your local Cook representative. This form is intended for US-based physicians only.
By clicking ‘Submit,’ you agree to the terms and conditions for collecting and processing your personal information, as included in our customer data privacy notice.

A bold decision: The ZILVER PTX randomized controlled trial was designed from the start to include 5-year follow-up data that would determine not only long-term benefits but also long-term safety signals or adverse events.
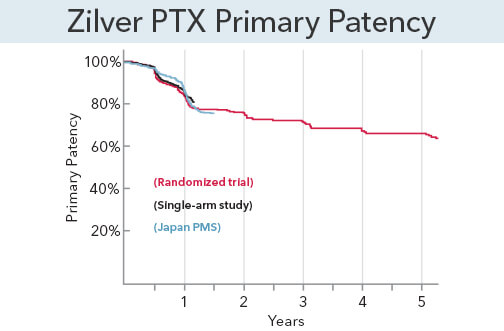
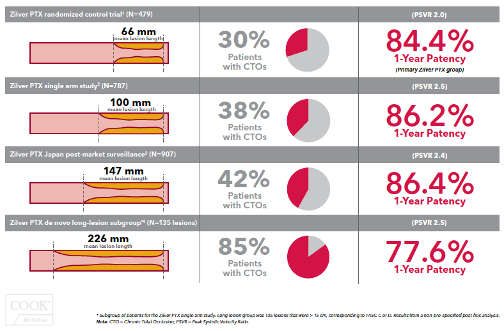
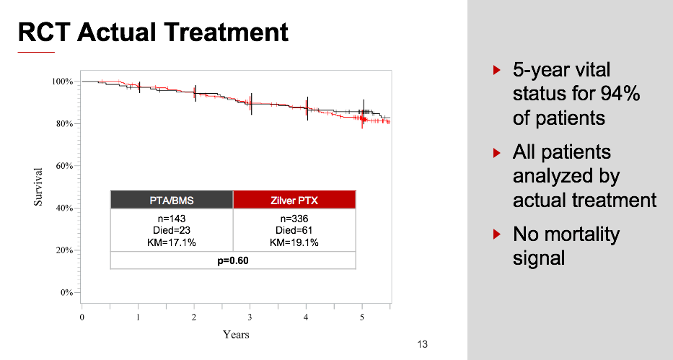
NOTE: Results are from the secondary randomization of ZIlver PTX vs. Zilver bare-metal stent.
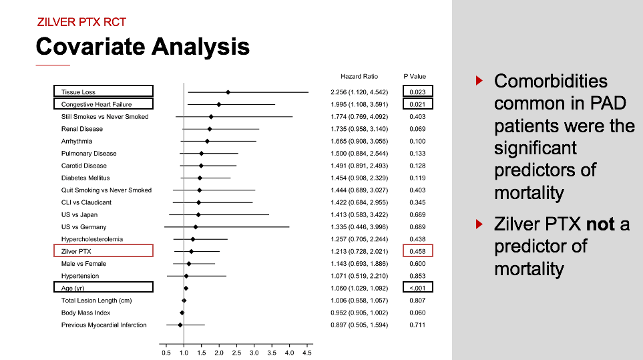
For some, controversies surrounding the use of paclitaxel-coated devices to treat PAD in the SFA have caused concern about patient safety. Transparency is the key to ensuring physicians have access to the safest and most effective technology available for treating PAD in a broad patient population. That’s why we took the unprecedented step of making our 5-year patient-level data fully available for the whole world to see.
See more of our updates on Zilver PTX global patient safety data.
The Zilver Flex platform has demonstrated fracture-resistant design through five years and has also demonstrated a lower fracture rate than the Eluvia DES.


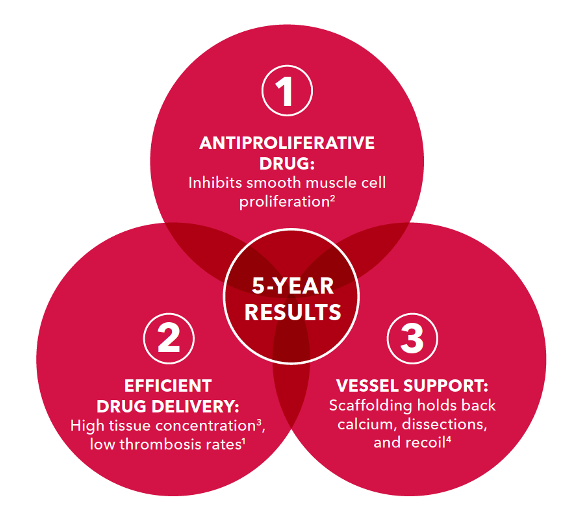
Only Zilver PTX combines an antiproliferative drug with efficient, polymer-free drug delivery and vessel support to demonstrate superior 5-year results against PTA and Zilver bare-metal stents.
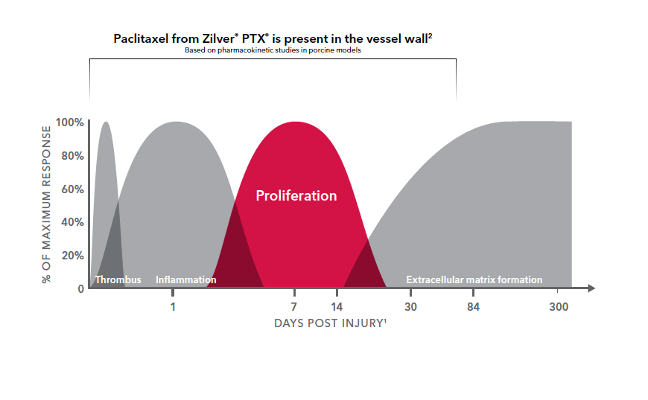
Short-term paclitaxel delivery in the SFA has been proven beneficial.
Zilver PTX is the only polymer-free SFA drug-eluting stent. Our proprietary, polymer-free coating process is shown to be safe and effective while eliminating the potential risks associated with permanent polymers.
More than 98% of the paclitaxel coating is released from the stent within 72 hours.1
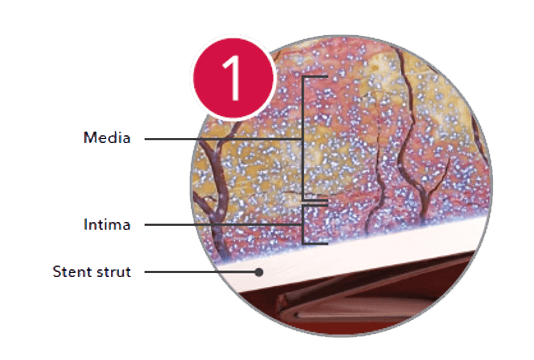
Paclitaxel remains in the artery for up to 56 days.1
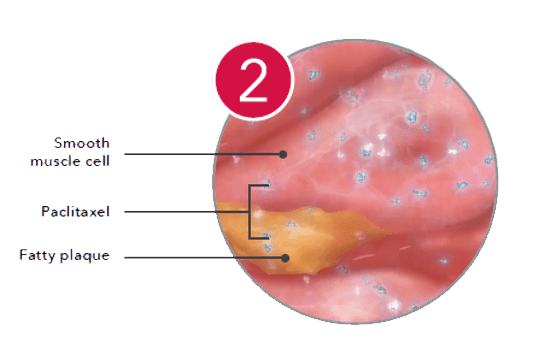
Inside the cell, the drug binds to microtubules and inhibits mitosis.1
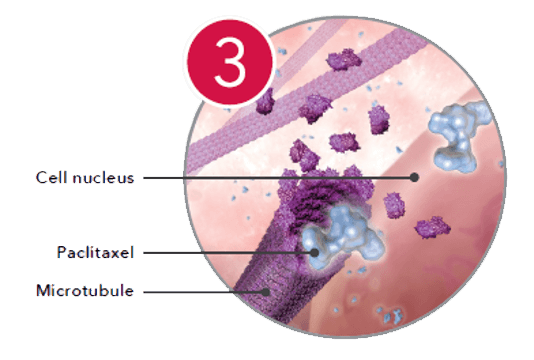
Zilver PTX offers twice the number of sizes and a longer indication than Eluvia.
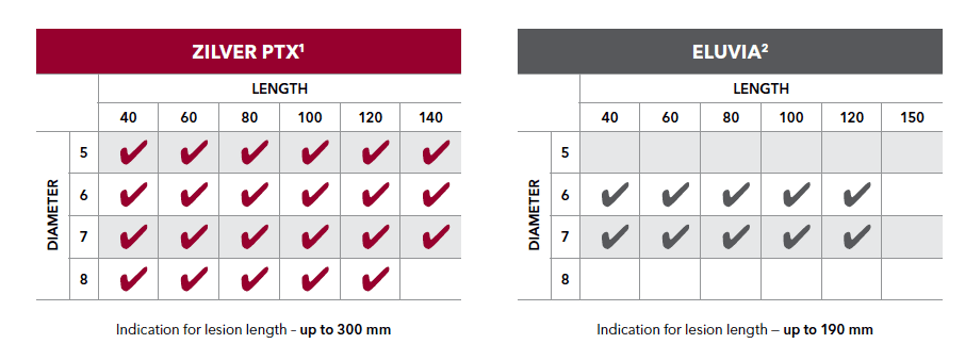
*Size availability as of November 2020
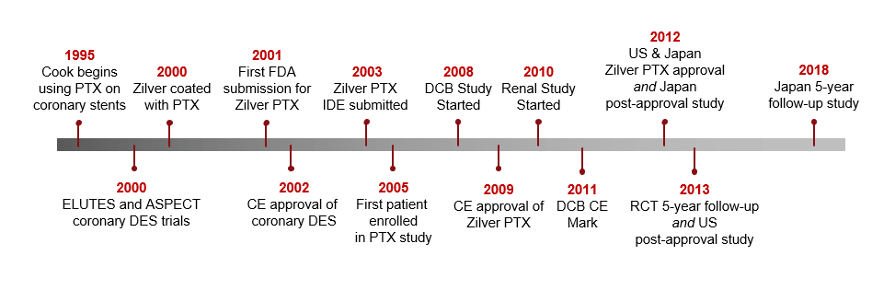
Zilver PTX started in the coronary and landed in the SFA.

This patient-focused site explains the causes, risk factors, symptoms, and treatment options for PAD.