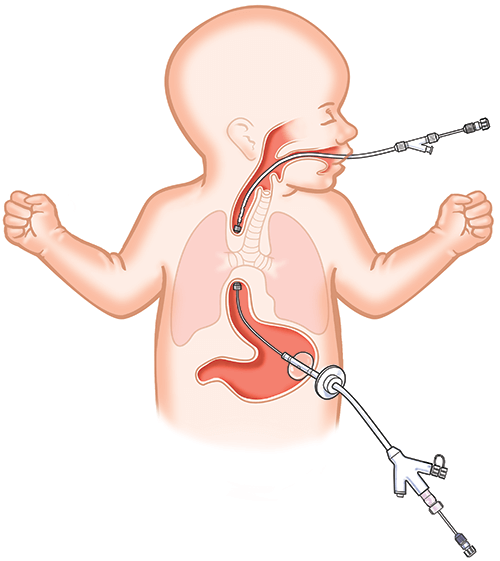
 Flourish Pediatric Esophageal Atresia Device
Flourish Pediatric Esophageal Atresia Device
Flourish was created to give doctors a minimally invasive alternative to surgery when treating esophageal atresia – a congenital birth defect which occurs in about 1 in every 2,500 newborns¹. Using rare earth magnets, Flourish gradually stretches both ends of the infant’s esophageal pouches together to form a fully functioning esophagus.
This device requires IRB approval: H150003
“Repairing My Baby’s Esophagus with Magnets” must be provided to caregiver prior to deciding treatment.
Resources:
Repairing My Baby’s Esophagus with Magnets
Flourish Pediatric Esophageal Atresia Device Datasheet
Flourish Pediatric Esophageal Atresia Device Instructions for Use
For more information on the Flourish Post Approval Study click here.
¹Mahoney L, Rosen R. Feeding Difficulties in Children with Esophageal Atresia. Paediatr Respir Rev. 2016 June; 19: 21–27. doi:10.1016/j.prrv.2015.06.002.
FOR SALE IN THE USA ONLY.
HUMANITARIAN DEVICE
Authorized by federal law for use in the treatment of lengthening atretic esophageal ends and creating an anastomosis with a non-surgical procedure in pediatric patients, up to one year of age with esophageal atresia without a tracheoesophageal fistula (TEF), or in pediatric patients up to one year of age for whom a concurrent TEF has been closed as a result of a prior procedure. The effectiveness of this use has not been demonstrated.