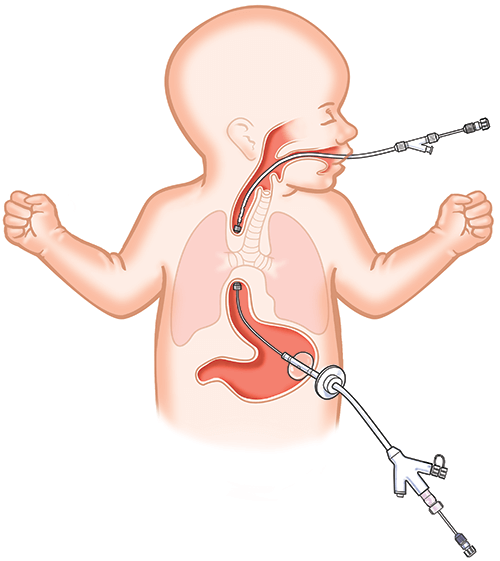
 Esophageal atresia (EA) is a rare anomaly, present at birth, characterized by an abnormally developed esophagus that is split into two separate parts that are not attached.1 There are a number of different types of esophageal atresia. Type C, when the upper section of the esophagus ends in a “blind pouch” and the lower section is attached to the trachea, is the most common type of EA.2 About 1 in every 4,100 newborns in the United States has some type of EA and/or tracheoesophageal fistula3 (when the trachea and esophagus are connected, as in EA type C; the two conditions frequently occur together).
Esophageal atresia (EA) is a rare anomaly, present at birth, characterized by an abnormally developed esophagus that is split into two separate parts that are not attached.1 There are a number of different types of esophageal atresia. Type C, when the upper section of the esophagus ends in a “blind pouch” and the lower section is attached to the trachea, is the most common type of EA.2 About 1 in every 4,100 newborns in the United States has some type of EA and/or tracheoesophageal fistula3 (when the trachea and esophagus are connected, as in EA type C; the two conditions frequently occur together).
How is EA treated?
Infants with EA are often easy to diagnose, as symptoms are usually detected soon after birth.2 X-ray imaging is typically used to confirm the diagnosis,1 and postnatal interventions are employed to ensure the baby’s safety until more permanent repairs can be made.4 Surgery to reconnect the two ends of the esophagus is the primary method of treating EA. This procedure is often completed soon after birth but may be delayed if an infant has additional congenital abnormalities or illnesses.5 Infants with EA often experience other abnormalities as well, including problems with the digestive system, heart, kidneys, or spinal column.6
Cook Medical driving innovations in EA treatment
The Flourish™ Pediatric Esophageal Atresia Device was created to give doctors a minimally invasive alternative to surgery when treating EA. Using rare earth magnets, Flourish gradually stretches both ends of the infant’s esophageal pouches toward each other until they connect to form a fully functioning esophagus.
For more information about Flourish, its Intended Use, and a clinical study on it, click here.
The Flourish Pediatric Esophageal Atresia Device requires IRB approval: H150003.
A copy of the brochure “Repairing My Baby’s Esophagus with Magnets” must be provided to the caregiver before a treatment decision is made.
FOR SALE IN THE USA ONLY
HUMANITARIAN DEVICE
Authorized by federal law for use in the treatment of lengthening atretic esophageal ends and creating an anastomosis with a non-surgical procedure in pediatric patients, up to one year of age with esophageal atresia without a tracheoesophageal fistula (TEF), or in pediatric patients up to one year of age for whom a concurrent TEF has been closed as a result of a prior procedure. The effectiveness of this use has not been demonstrated.
SOURCES
- Facts about esophageal atresia. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/ncbddd/birthdefects/esophagealatresia.html. Accessed January 12, 2021.
- Esophageal atresia. Boston Children’s Hospital Web site. https://www.childrenshospital.org/conditions-and-treatments/conditions/e/esophageal-atresia. Accessed January 12, 2021.
- Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019;111(18):1420–1435.
- Scott DA. Esophageal atresia / tracheoesophageal fistula overview. GeneReviews® Web site. https://www.ncbi.nlm.nih.gov/books/NBK5192/. Published March 12, 2009. Updated September 20, 2018. Accessed January 12, 2021.
- Esophageal atresia and/or tracheoesophageal fistula. NORD® (National Organization for Rare Disorders) Web site. https://rarediseases.org/rare-diseases/esophageal-atresia-andor-tracheoesophageal-fistula/. Accessed January 12, 2021.
- Chittmittrapap S, Spitz L, Kiely EM, et al. Oesophageal atresia and associated anomalies. Arch Dis Child. 1989;64(3):364–68.